Our Science
SP1707
SP1707 is the development name/number of the Company's first drug candidate undergoing clinical development that contains the active ingredient used by Dr. Alan Shackelford in our initial targeted epilepsy population. SP1707 is being developed, ultimately, as a proprietary oral cannabinoid formulation that is amenable to being dosed once daily in patients.
Pre-Clinical Development and Real-World Patient Experience
SP1707 underwent a series of pre-clinical trials to test its effectiveness in epilepsy animal models and was successful at achieving results that warranted this drug candidate being moved forward in the development process.
The Company also evaluated patient data from Dr. Shackelford's real-world experience and discovered that similar human dose equivalents effective in the Company's animal trials were also effective at significantly impacting patients suffering from epilepsy in many patients. The Company is initially focusing on a specific seizure type for its first indication for SP1707. This indication is backed by ~356 patient treatment years of data.
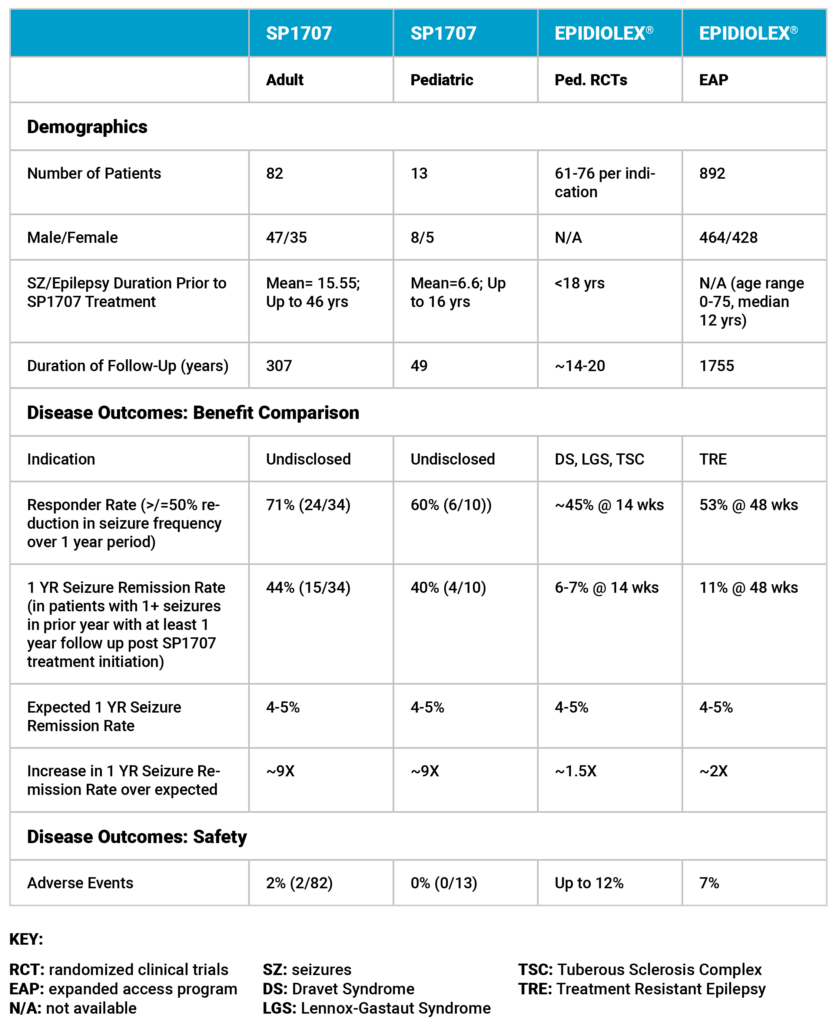
Dr. Shackelford's real-world patient experience shows that in adult and pediatric patients with a specific seizure type, patients taking his cannabinoid-based treatment for at least one year resulted in clinically significant positive outcomes. The analysis of results (as of January 12, 2023) is shown in the table below. Published data from Jazz Pharmaceuticals plc.’s cannabidiol (“CBD”) based product, EPIDIOLEX®, is shown as a comparator.
Dr. Shackelford’s Real-World Experience Compared With Published Data from Jazz Pharmaceuticals plc.